Microbial Identification
Trace Analytics is a third-party laboratory that processes thousands of microbial samples a year. Microbial identification is an important process of any microbial monitoring plan. Whether you’re looking to meet compressed air microbial limits, or have an environmental monitoring plan to adhere to, Trace can help you with identifying the microorganisms in your facility.
Microbial Identification – Phenotypic ID
Each day people are exposed to millions of microorganisms in the air and surfaces. As humans carry 1012 microorganisms on their skin and 1014 microorganisms in their gut, we might be one of the greatest sources of contamination in a facilities environment. Consequently, regularly testing the ambient air, compressed air, and surfaces in your facility is the first step in building a robust microbiological monitoring program. The more robust the program, the better equipped the facility is for microbial alert or alarm level remediation. Identifying these organisms allows the user to pinpoint problem areas and create a tailored remediation plan.
Service Info
Microbial Contamination Tips
WHY DO FACILITIES WANT TO IDENTIFY MICROORGANISMS?
The most commonly used forms of microbiological analysis in air or surface monitoring are detection, enumeration, and identification. Identifying microorganisms that are found in areas or concentrations at or above alert levels can provide insight into cleaning and sanitization protocols that may be inappropriate or insufficient. Regular monitoring of microorganisms allows manufacturing facilities to ensure procedures are working efficiently and their end-products are protected. The identification of indicator organisms can pinpoint problems or issues within the facility or health and safety protocols. Compressed air microbial testing can help protect end products from contamination.
For instance, finding aerobic, or oxygen requiring organisms, in an anaerobic environment may indicate that there is an ambient air or oxygen leak in that environment, or that aseptic technique training has failed. Finding coliforms or potential pathogenic bacteria in food or pharmaceutical manufacturing plants provides facilities with the opportunity to take preventative action to protect not only their bottom line, but reputation as well.
Microorganisms’ pervasive nature allows them to adapt and utilize what is readily available to them. Some are even able to consume plastics, rust, and oil for energy so a compressed air system or manufacturing facility could act as a hospitable environment to any number of species, particularly if the safety protocols are not working to standard.
WHAT TYPES OF MICROBIAL IDENTIFICATION ARE AVAILABLE?
Trace Analytics offers a variety of microbial analyses including Total Aerobic Plate Count, Total Anaerobic Plate Count, Total Fungal Count, Gram’s staining, chromogenic identification, and phenotypic identification. When a customer is testing to a standard where the number and cell shape (presumptive identification) of the microorganism is no longer sufficient information, confirmatory identification testing can be applied. The Biolog microbial identification system can provide additional answers for users looking for more information about their found microbe.
Each genus and species of organism has specific sensitivities to certain chemicals, enzymes, and drugs, as well as the ability to metabolize certain compounds. Trace Analytics can test for different properties exhibited by varying bacteria, yeast, and filamentous mold to “fingerprint” the organism. Our 17025 accredited laboratory is able to identify within its fingerprint library of over 2,900 species. The Biolog system was created specifically for applications in quality control and environmental monitoring – focusing on microorganisms that are more prevalent and pertinent outside of the clinical setting.
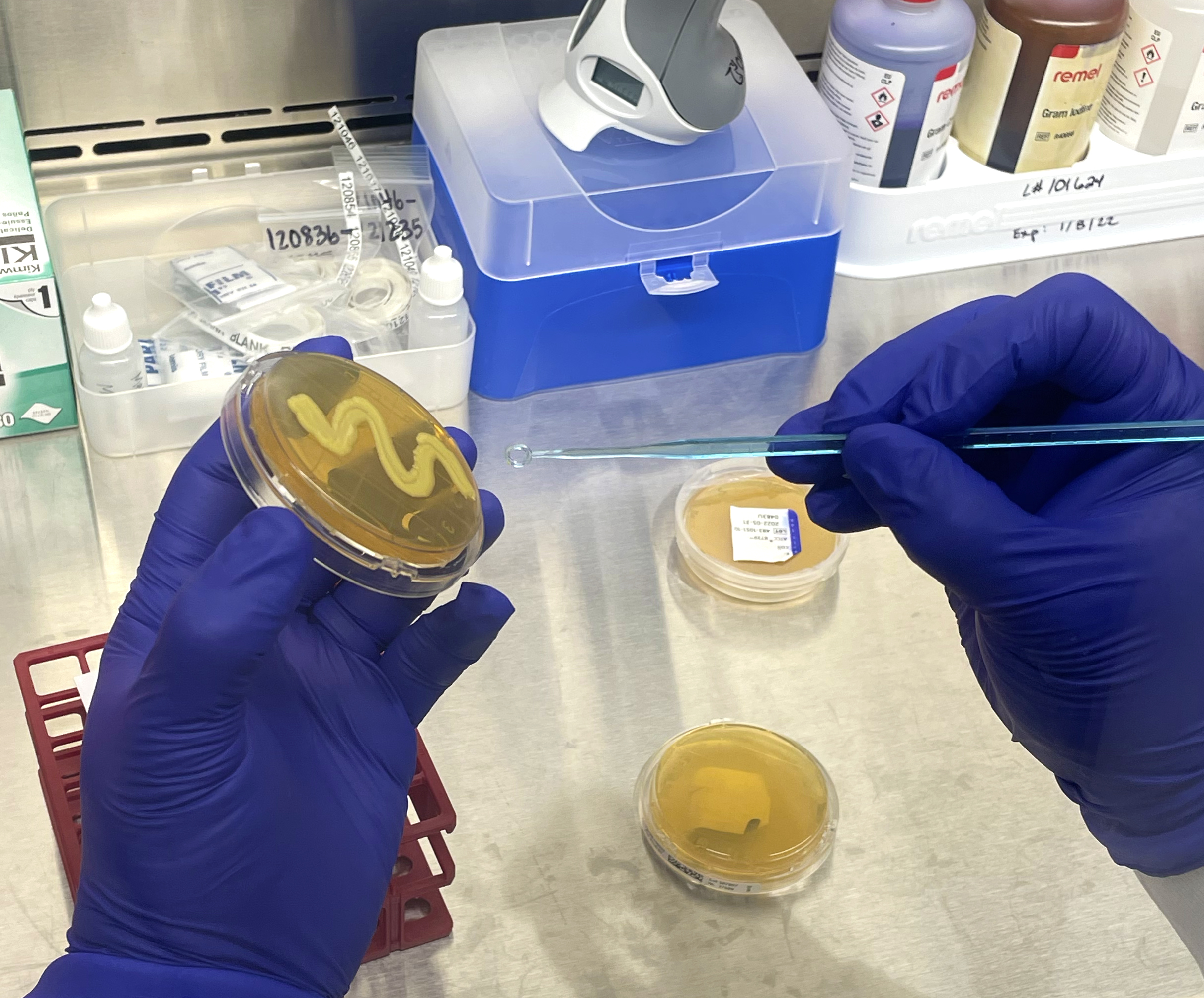
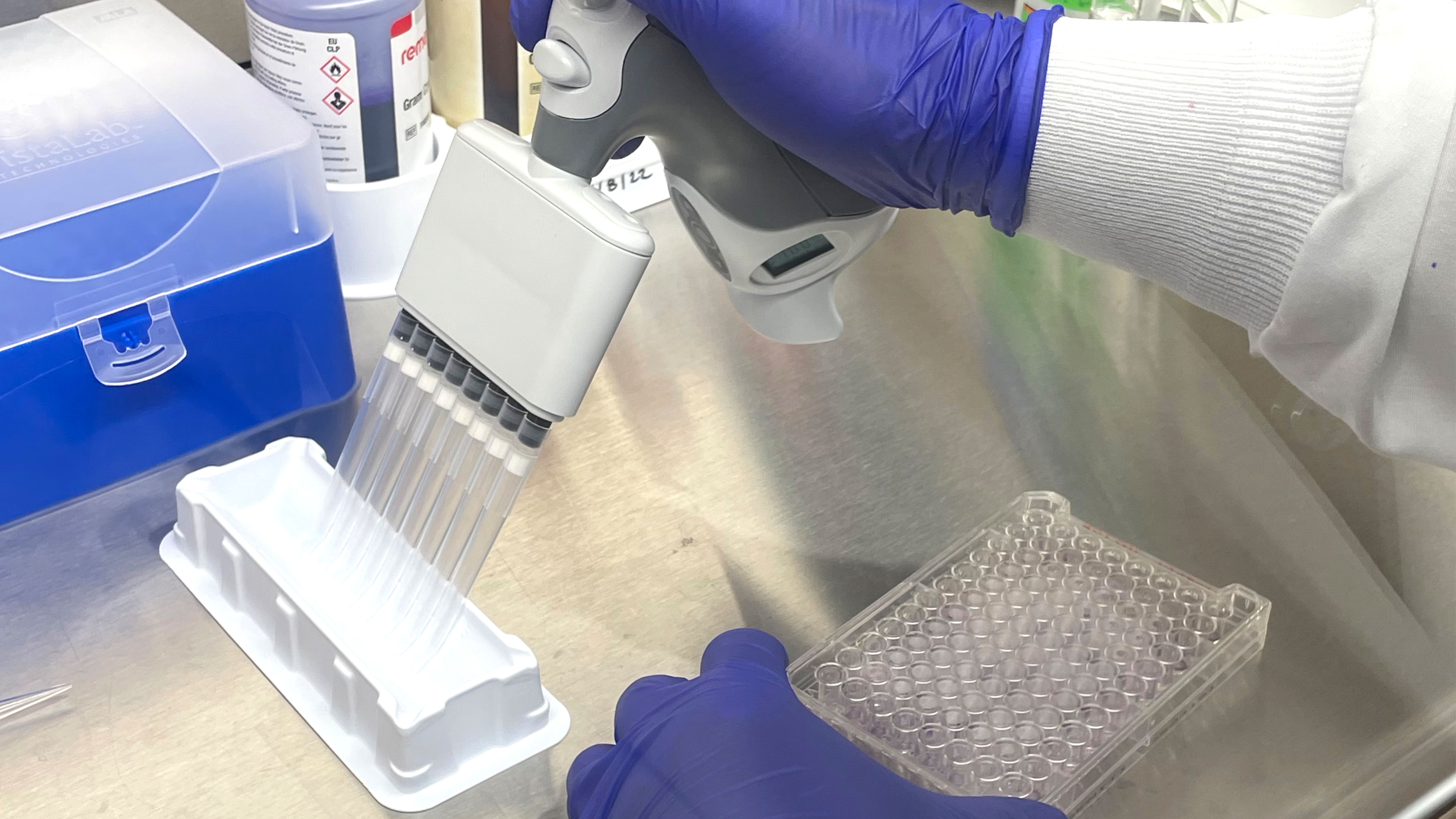
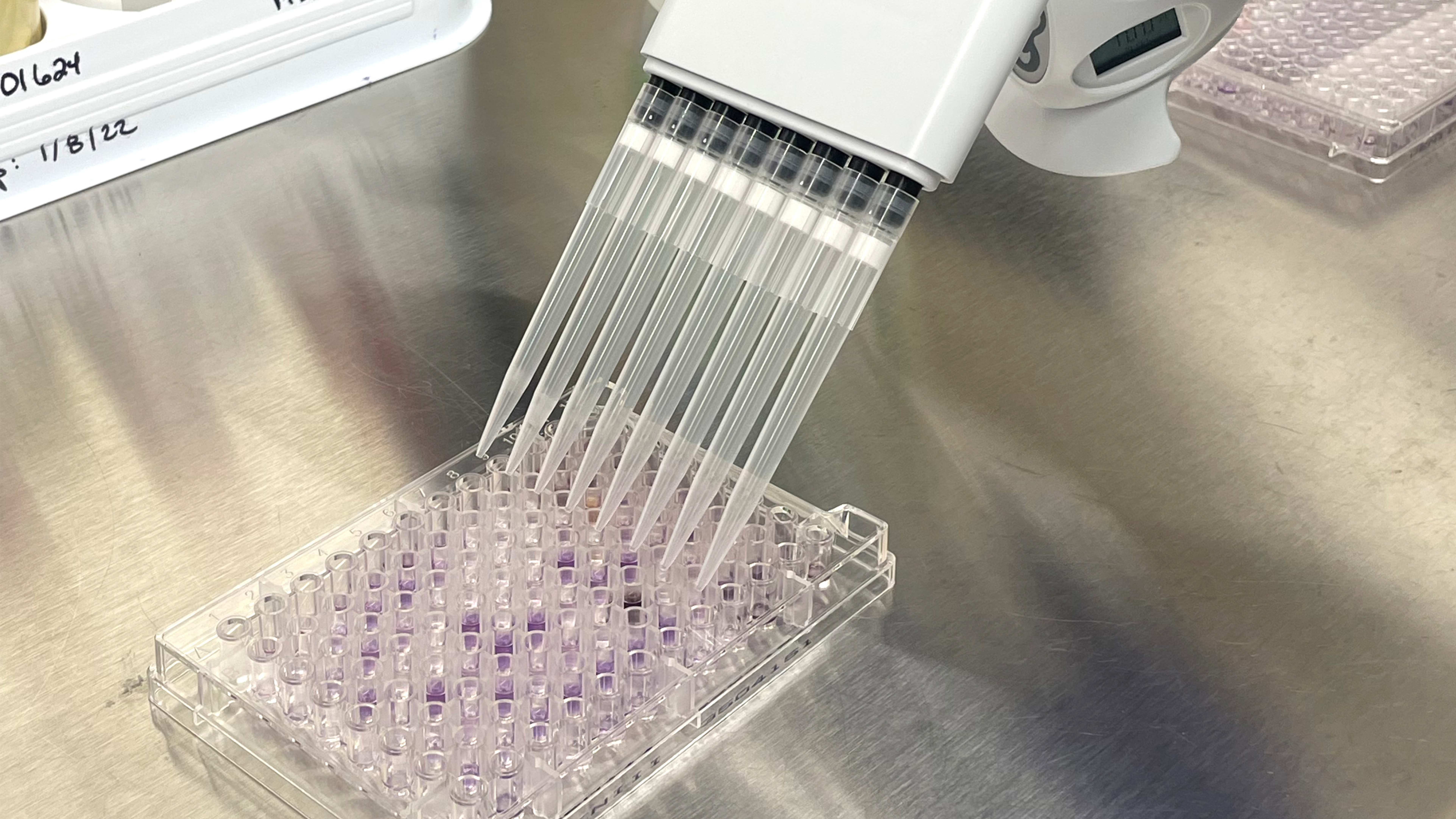
The Biolog testing process is streamlined and straightforward. The organism is first obtained or may be re-streaked on a different plate for culture isolation and viability purposes. Once isolated, the pure culture is transferred into an inoculum fluid, mixed well, and then inoculated into each well of the MicroPlate. The plate is incubated prior to analysis on the Biolog, with time and temperature depending on the microorganism. The Biolog is installation, operation, and performance validated using standard reference microorganisms certified by the accredited American Type Culture Collection. The Biolog is also 21 CFR Part 11 compliant, a code by the Food and Drug Administration regulating electronic records and signatures for audit trail traceability purposes.
Confirmatory testing is required for select environmental monitoring microbial specifications like USP 797. Compared to presumptive testing, which identifies only present or not detected rather than true identification, results can provide multifaceted detail on what microorganisms are present in your facility or compressed air system.
The Biolog identification system can be applied to microorganisms gathered using a variety of sampling methods including:
- Pinocchio Super I or II for compressed air systems
- Trio.bas Mono for ambient air samples
- Swabs/contact plates for surfaces
For more information on sampling for microorganisms, click below.
Then return the samples to Trace Analytics using the provided Next Day shipping label. Once on-site, the samples will undergo incubation in our state-of-the-art accredited laboratory. Biolog identification services are available once microbial growth appears. Your customer service representative will alert you if the plates have microbial growth and you can add microbial identification to your order.

Whether identifying microbes within a compressed air system, on surfaces in a facility, or in the ambient air, Trace Analytics can facilitate your microbial testing.
A2LA accredited to ISO 17025:2017, Trace Analytics is a third-party laboratory that processes thousands of samples a year. Contact us for more information or to set up your facility’s monitoring plan.
HOW BIOLOG IDENTIFICATION ANALYSIS WORKS
Phenotypic identification is performed with the Biolog at Trace Analytics. The Biolog test plate (MicroPlate) has 23 chemical sensitivity assays, 71 cellular carbon source utilization confirmatory tests, and two controls to make up each specific phenotypic fingerprint. Each type of microorganism obtains different or varying levels of carbon sources for development and reproduction. That, in addition to the chemical sensitivity tests, provides each microorganism with a unique, individual pattern. Being able to identify microorganisms to the genus and species level can be helpful to identify not only indicator organisms but also assist in troubleshooting potential problems. The Biolog can provide much more information than Gram’s staining or ATP transfer activity.
The Biolog testing process is streamlined and straightforward. The organism is first obtained or may be re-streaked on a different plate for culture isolation and viability purposes. Once isolated, the pure culture is transferred into an inoculum fluid, mixed well, and then inoculated into each well of the MicroPlate. The plate is incubated prior to analysis on the Biolog, with time and temperature depending on the microorganism. The Biolog is installation, operation, and performance validated using standard reference microorganisms certified by the accredited American Type Culture Collection. The Biolog is also 21 CFR Part 11 compliant, a code by the Food and Drug Administration regulating electronic records and signatures for audit trail traceability purposes.
Confirmatory testing is required for select environmental monitoring microbial specifications like USP 797. Compared to presumptive testing, which identifies only present or not detected rather than true identification, results can provide multifaceted detail on what microorganisms are present in your facility or compressed air system.
TERMS AND DEFINITIONS
TOLL FREE
1.800.247.1024
LOCAL LINE
512.263.0000
OFFICE HOURS (CT)




