New installations, add-ons, and modifications of compressed air and gas systems should all be validated prior to use. Air or gas system validations become even more critical in the cases of pharmaceutical or food manufacturing facilities with high-risk end-products. In order for a validation to be successful, the facility must designate specifications, timelines, documentation, and training. The experts at Trace Analytics are here to provide guidance on setting up a successful compressed air or gas system validation.
Specs and Testing:
According to the ISPE (International Society of Pharmaceutical Engineers), Good Practice Guide for Process Gases, compressed air systems and process gases should be free from contamination and regularly tested (2016). Testing for particles, water, oil, and microorganisms is an important part of any compressed air or gas system validation.
ISO 8573 is the international standard for compressed air and provides purity limits and analytical methods. Trace Analytics’ analyses and sampling kits meet ISO 8573 requirements. Download the risk assessment to help assess the appropriate purity limits and testing requirements for your unique system.
Process Air/Gas Contaminants:
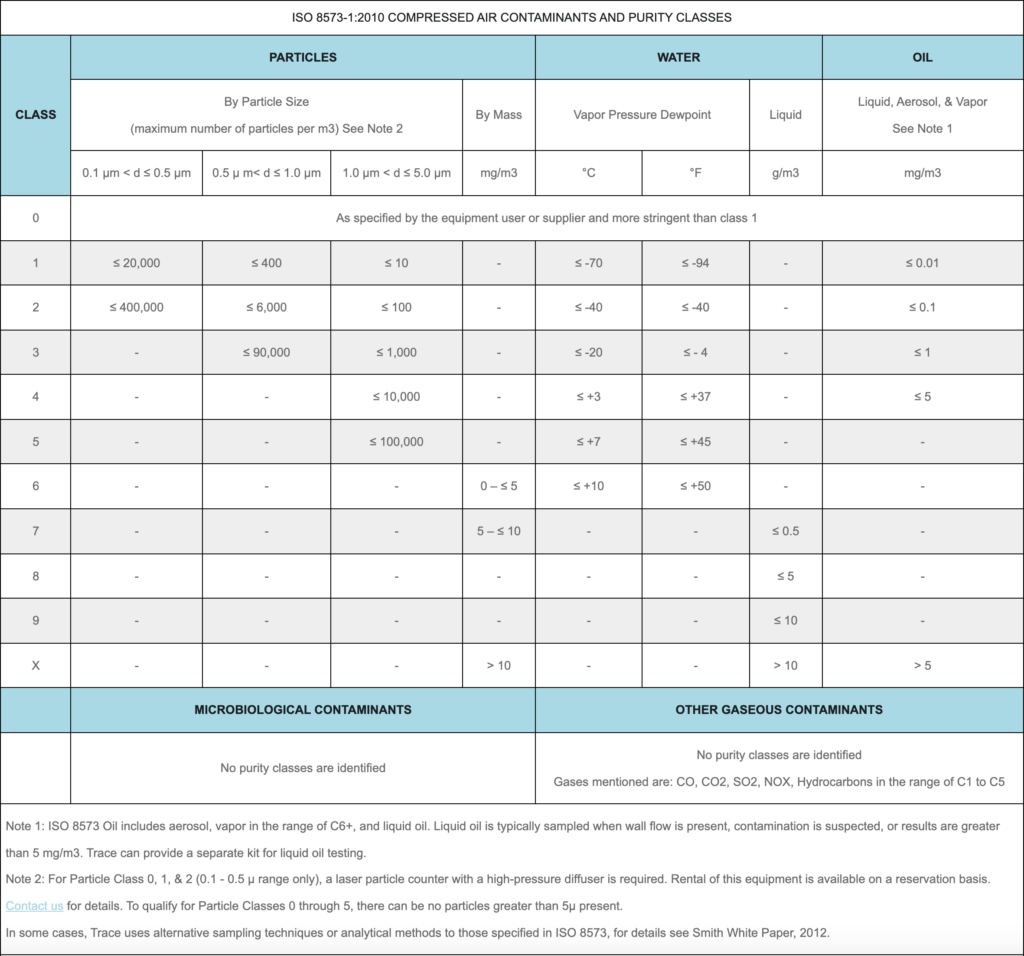
Particulates can be analyzed in a couple of different ways: sizing and binning (microscopy), measurement by mass (gravimetry), or via laser particle counter. ISO 8573-1 outlines the acceptable size ranges and limits in the purity class chart below.
Water vapor testing can be performed on-site with Draeger tubes to determine if filtration is working as expected.
Total oil is defined as oil aerosol and oil vapor by ISO 8573. Depending on your specification requirements, you may or may not need to test for oil vapor. ISO 8573 Classes 1 and 2 require total oil results, while oil classes 3 and 4 require only aerosol results. If your specification requires vapor testing, Trace provides additional identification of vapors. This can be helpful in troubleshooting or tracking down any contamination that may be present.
Non-viable particulates can be analyzed by Total Plate Count, Gram’s staining, or presumptive identification. Trace can test to specifications like ISO 14644 and ISO 14698 for microorganisms.
Gas purity can be an important part of a validation process as well. It’s essential that any pure gases like nitrogen, oxygen, or argon be produced at the level of quality required for the end-product.
Process Air/Gas Validation Timelines:
It is important to adequately schedule your sampling timelines in order to receive results when you need them. Most validations require passing results for a few days in a row. Rush results are available from an accredited laboratory like Trace Analytics which can provide next day reports. This can prevent the need for travel back to resample. Immediate feedback can also be achieved by using a Laser Particle Counter for particulate contamination. Water vapor tubes can alert to issues with water contamination.
Depending on the purity classes required, some sampling times may be longer than others. Refer to your experts at Trace Analytics for appropriate sampling times and be sure to schedule your sampling days accordingly.
Successful Sampling of Process Air/Gas
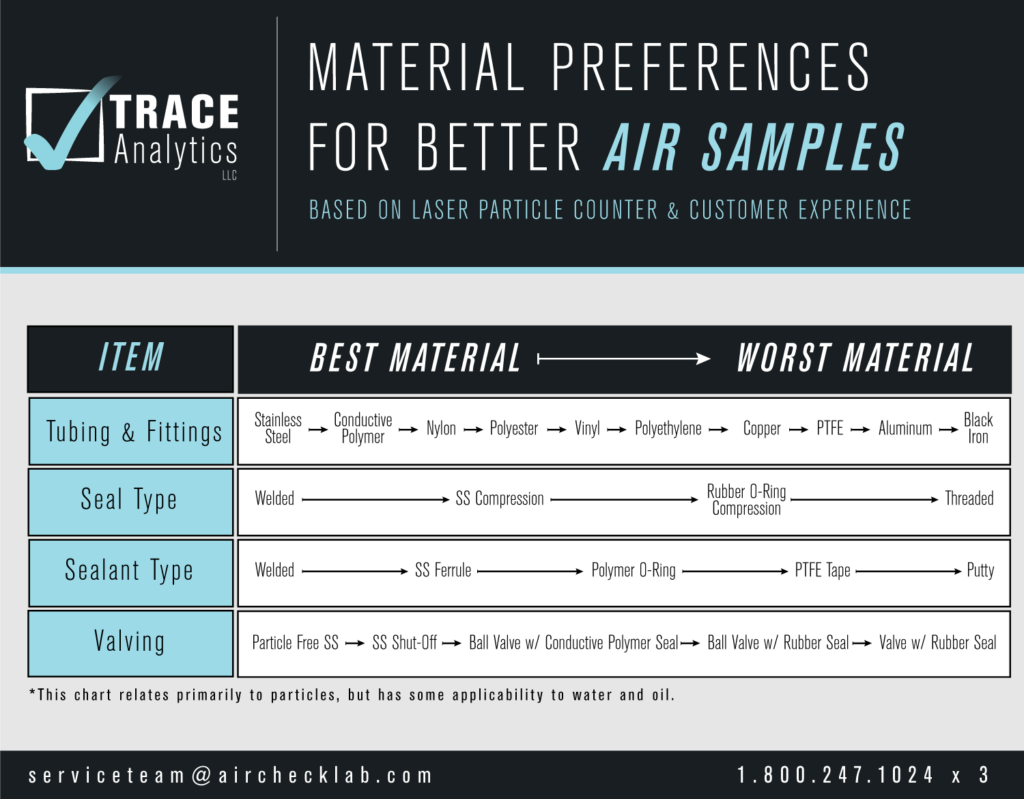
The materials used in the sampling set up are critical to a new system validation. Refer to our helpful chart for best material choices.
Documentation
Another major requirement for any compressed air or gas system validation is documentation and training. Trace Analytics will provide you with online training, course completion certificates, chains of custody, calibration certificates, and analysis reports. To view examples of sample reports, click here.
Conclusion
Validations can be complicated processes, but with the help of the experts at Trace Analytics, users will find they have everything they need to successfully test for contaminants and purity in the qualification or validation of a compressed air or gas system.
Read the original article on Pharmtech.com here.