Compressed air, also referred to as process gas, is used in many capacities in the pharmaceutical industry. Regular quality control testing plays an important role in the safety of your products. The ISPE Good Practice Guide for process gases asserts that compressed air usage in pharmaceutical manufacturing should be free from contaminants and routinely maintained and tested. Although there are not specific limits set, the ISPE Good Practice Guide does provide helpful suggestions, recommendations and guidelines for adequate system design, appropriate controls and routine maintenance of compressed air systems. Analytical and risk-based testing methods are recommended for all pharmaceutical process gases and compressed air systems (ISPE, 2011).
Put ISPE Good Practice Guide into Action
The first and most critical step toward implementing the Good Practice Guide is to assess the risks associated with both your system and your product. It is important to consider the role that compressed air plays in your facility and the interaction that the process gas has with the product. A facility must employ adequate controls for moisture, total hydrocarbons, particles, and microorganisms with a regularly scheduled maintenance plan.
Perform a Risk Assessment
When performing a risk analysis for a pharmaceutical compressed air system, you’ll need to determine the potential contaminants, identify the sampling points, and determine the appropriate process parameters for each control sample point.
Systemic risks can include piping, storage, point of use, filtration, and dryers. These are all critical aspects that should be carefully identified and documented according to ISPE Good Practice Guide (2011). Each facility will have unique risks based on location, environmental factors, equipment, operations and system age. Each piece of the system that comes into contact with the compressed air should be considered.
There are a variety of ways that a compressed air system can be set up, but most systems include a compressor, filtration mechanisms, air receivers, dryers and controls. Each of these components will come with manufacturer recommendations for care and maintenance. In order to ensure the systems are working properly, take care to follow these instructions closely. The ISPE Good Practice Guide (2016) states that sample points should be regularly maintained and should not introduce a risk of contamination to the system. Completing a risk assessment can help account for each of these factors and determine the appropriate purity classes for your compressed air.
Evaluate what in your system might go wrong, what probably will go wrong, and the potential consequences of these risks (ISPE, 2011). Part of an effective risk assessment is to determine what can be done to eliminate these risks and protect the end user.
Needs Vary Based on Product Risk
When considering product specific risks, you should analyze the components, ingredients, and specifications of your product. Depending on the amount and type of contact that the product has with the compressed air, these risks will vary. Direct contact increases the risks of contamination because the air is directly impacting the product. Indirect contact means that lower purity classes may be acceptable.
According to ISPE Good Practice Guide (2016), compressed air can be used to dry a product, spray paint a product, move a product, or could even be included as a component or ingredient. Analyzing your product’s relationship with compressed air will allow your facility to determine appropriate purity class levels.
Equally important is the consideration of hazards to a product. According to the US FDA Guidance for Industry Sterile Drug Products Produced by Aseptic Processing – Current Good Manufacturing Practice (2004), “a compressed gas should be of appropriate purity (e.g., free from oil) and it’s microbiological and particle quality after filtration should be equal to or better than that of the air in the environment into which the gas is introduced”. This FDA standard is adopted by many in the pharmaceutical industry, as it equates cleanroom or environmental standards to the compressed air. It is important to understand the product’s specific needs and the dangers that particles, water, oil or microorganisms present.
Many pharmaceutical companies also follow ISO 8573 which is an international standard that provides additional guidelines and recommendations. For more information on ISO 8573 specifications and clarification, see this article.
Compressed Air Quality Testing for Contaminants
Sampling your compressed air does not have to be a difficult process. Examine the type of gas, the needs of the product manufactured, the regulatory controls in place, and the potential hazard to the user (ISPE, 2011). Bringing together all of these considerations will allow you to effectively choose the best testing equipment. An accredited third party laboratory can equip you with simple sampling tools and then provide a compressed air analysis quickly and efficiently.
Particles: Depending on the purity class that your facility is trying to achieve, a few different methods are available when testing for particulates. In order to achieve Purity Classes 0, 1 or 2, a laser particle counter should be used. If Purity Classes 3-6 are required, then sample collection by filter will be sufficient.
Microorganisms: The best way to test for microorganisms in a compressed air system is with an impaction sampler such as the SAS Pinocchio Super II KX00. The sampler collects microorganisms on an agar plate and the contamination is then analyzed using total plate count and bacterial classification.
Water Vapor: Facilities can test for water vapor contamination using chemical reactor tubes. It is important to follow the manufacturer’s recommendations when testing with this media, but overall, it is a simple and inexpensive way to ensure your compressed air is free from moisture.
Oil Aerosol: As with particles, oil aerosol can be collected with a filter cassette membrane. It is important to ensure that oil aerosol does not collect and pool in the compressor system.
Oil Vapor: Similar to water vapor, oil vapor can be tested using a detector tube. Charcoal tubes are best for sampling for oil vapor.
Create a Plan for Contamination Prevention
As previously mentioned, a compressed air system should be equipped with the necessary preventative measures including, filters, dryers, traps, and sampling ports. Most compressed air systems are designed, controlled, and maintained in a dry state to prevent the growth of microorganisms. Additionally, oil free compressors can allow facilities to prevent against the pooling of oil in their systems. Fittings, tubes, and piping should not introduce risk of contamination. It is recommended that these tubes be carefully chosen so as to not shed particles or encourage the pooling of water or oil.
Ideally, sampling locations should be after the final filter so all the particles, water, oil, and microorganisms have been removed. This sample will be most representative of the air coming into contact with your products and should meet your facility’s specifications. The end of the distribution line is another important point for sampling. Should the piping release contamination throughout the line, air quality may be much worse at the end of the distribution line. All critical points of use should also be sampled to ensure proper air quality, as they have direct impact on product quality.
Occasionally, manufacturers fail audits and tests not because of the actual air quality, but because of a lack of regular maintenance, or ineffective materials. Troubleshoot your system using this informational webinar. Regular compressed air analyses will help to provide historical data and ensure that your system maintains a satisfactory level of quality over time.
Monitoring Your Compressed Air Quality
According to the ISPE Good Practice Guide, determining sampling plans and monitoring schedules will be up to each individual facility. Keep in mind that the more complex the compressed air system, the more sampling points required for quality control testing. ISPE Good Practice Guide (2016) recommends the following sampling philosophy:
- Commissioning – This type of sampling acts as a prequalification and is recommended mainly for gas generation and distribution systems.
- Initial Performance Qualification – This sampling allows pharmaceutical manufacturers to verify the system is clean, ensuring that no contaminants are present before beginning production.
- Requalification – Refers to sampling after any major or minor changes have been made to the system. This includes additions to the piping system, repairs to valves or system alterations.
- Routine Monitoring – It is required that compressed air at least meets the facility’s cleanroom or environmental classifications. The frequency of quality control testing is often based on an individual risk assessment. For example, testing can be quarterly, twice a year, or before and after maintenance.
ISPE Good Practice Guide recognizes that compressed air quality testing approaches may vary between pharmaceutical companies, but it is critical that these variations exist based on the specific products, system, and risks.
The following chart is a helpful indication of when to test for what contaminants.
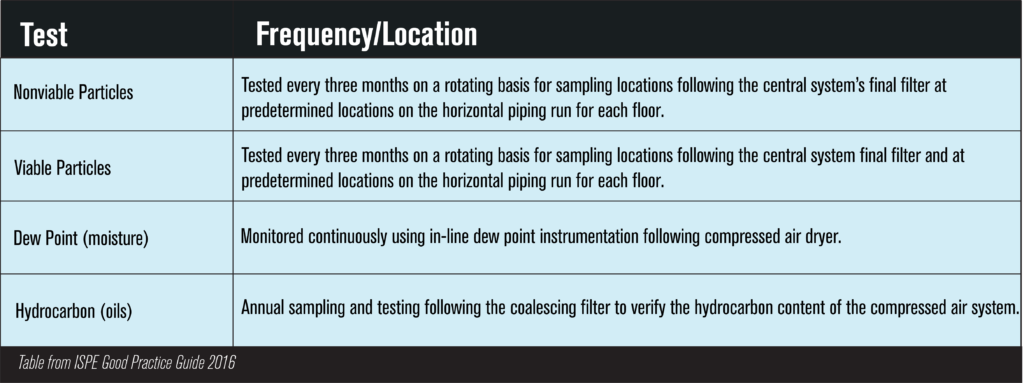
Another important aspect of a quality plan is to work with a third party, accredited laboratory like Trace Analytics. Offering a variety of specifications, Trace can help ensure that your air is free from contaminants and safe to use on your products. Ensuring the quality of your pharmaceutical process air is simple and effective when using the ISPE Good Practice Guide along with your facility’s risk assessment.
References:
1. Hagopian, Brian, et al. Good Practice Guide: Sampling for Pharmaceutical Water, Steam and Process Gases, ISPE, 2016.
2. Larrabee, Chad, and Nicholas Haycocks. ISPE Good Practice Guide: Process Gases. ISPE, 2011.
3. ISO 8573-2010: http://www.airchecklab.com/resources/standards-specifications/iso-8573-1-2010-compressed-air-specifications/










