Compressed air is often referred to as the “fourth utility” in manufacturing—vital yet frequently overlooked. Whether you’re in food processing, pharmaceuticals, beverage production, or packaging, compressed air is everywhere. If not properly maintained and monitored, it can spread contamination to end products. Putting products, brand reputation, and consumer safety at risk.
During a recent IFSQN webinar hosted by AirCheck expert Erin Zimmerman, attendees were given a deep dive into compressed air monitoring, common sources of contamination, and how to ensure compliance with industry standards like ISO 8573 and SQF. Here’s what you need to know.
Why Compressed Air Monitoring Matters
Compressed air and gases can directly contact food, packaging, or equipment, meaning they can be a significant source of contamination. Contaminants such as particles, water vapor, oil aerosols, oil vapors, and microorganisms can compromise product safety and quality if left unchecked.
Standards like ISO 8573 and guidelines from regulatory bodies such as SQF and BRCGS are in place to ensure that manufacturers maintain clean, dry, and safe air. However, awareness of these risks is still relatively new in some industries.
Understanding ISO 8573
ISO 8573 is the international standard for compressed air quality. ISO 8573-1 defines purity classes for particles, water, and oil, with Class 1 being the strictest and Class 6 being the most lenient. It provides a “common language” for air quality testing between manufacturers, OEM providers and laboratories.
ISO 8573-7 outlines methods for testing microorganisms, though it does not include a defined purity class chart for microbial contaminants. This makes regular risk-based assessments even more crucial.
The Four Main Compressed Air Contaminants
1. Particles
Particles in your compressed air system could originate from external intake, degraded piping, tape, or even the product itself. Even microscopic particles invisible to the naked eye can contaminate end products. Materials like black iron piping are major culprits in particle shedding and should be replaced or protected with proper filtration.
2. Water Vapor
Water vapor is introduced via permeable tubing, system intakes, and inadequate dryers. Moisture promotes microbial growth and corrosion. Ensuring proper filtration and knowing your dryer’s capabilities are key. Liquid water or visible condensation is a sign that your system is not adequately removing moisture.
3. Total Oil: Oil Aerosol and Vapor
Even “oil-free” compressors aren’t immune to oil contamination, as oil vapor can enter through intake air contaminated with solvents or exhaust. Poorly maintained lubricants, hoses, and fittings also contribute to contamination. ISO 8573-1 testing should cover both oil aerosol (mist) and vapor for comprehensive analysis.
4. Microorganisms
Microorganisms—fungi, bacteria, and mold—can be introduced via human contact, ambient air, or moist surfaces. Food and pharmaceutical industries are particularly vulnerable. Aseptic sampling techniques are essential to receive accurate and reliable results. Regular microbial testing per ISO 8573-7 necessitates specialized equipment like impaction samplers. Make sure that the sampling methods you choose are validated, controlled, and appropriate per ISO 8573 requirements.
Choosing the Right Equipment
Trace Analytics offers tailored solutions through its AirCheck kits, such as:
•K810 AirCheck Kit for particles, water, and oil
•Laser Particle Counters (LPCs) for high-precision particle detection
•Pinocchio Impaction Sampler for microbial testing
•K802 Kit for gas purity sampling
Each comes with detailed instructions and video guides, empowering users to collect samples confidently and correctly.
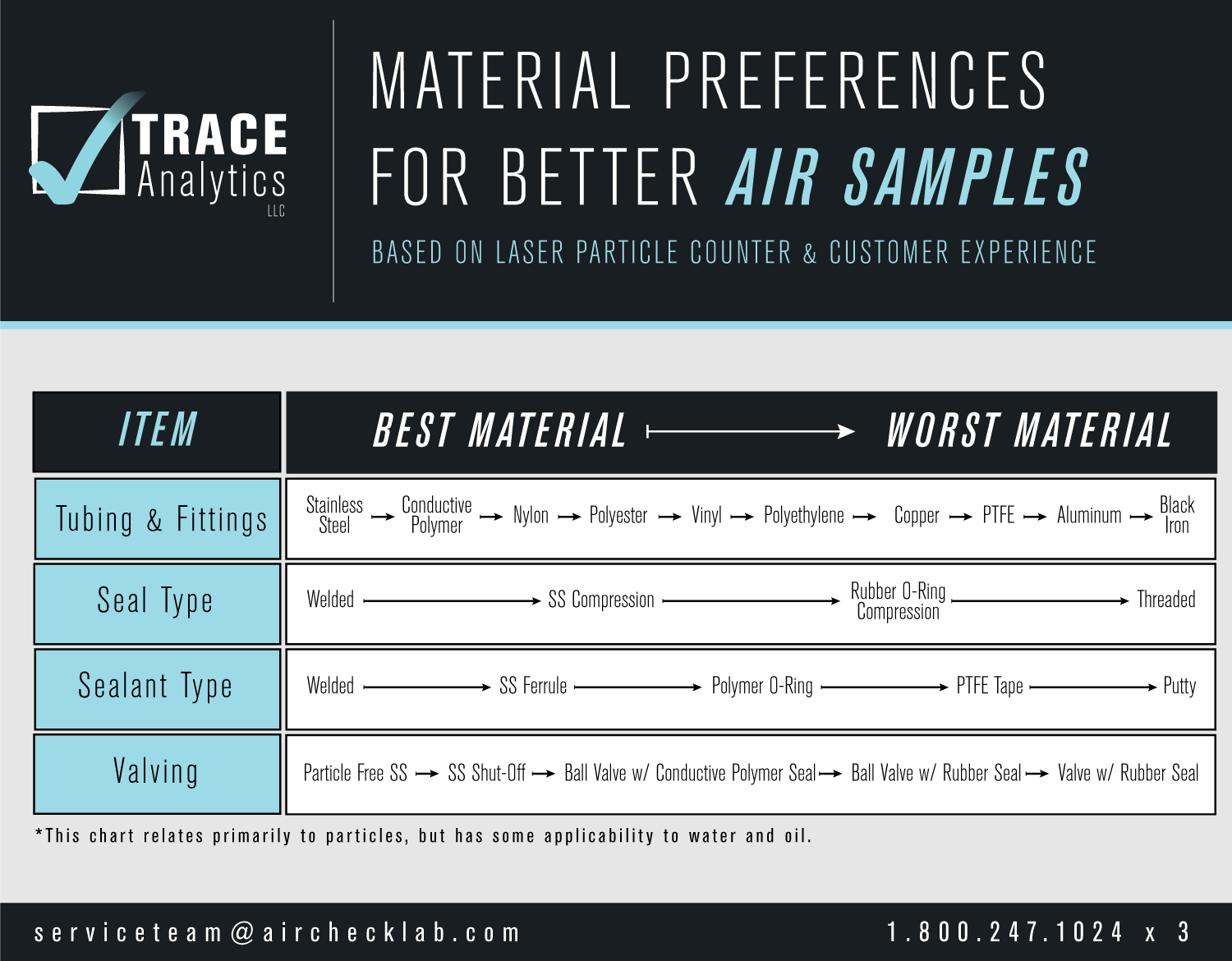
Best Practices for Contamination free Compressed Air Systems
Choose particle-free hoses and avoid excessive hose lengths
•Replace aging rubber and plastic materials regularly
•Select hard metals (e.g., stainless steel) over particle-shedding options like copper or black iron
•Welded sealants are preferred over tapes or putties
•Avoid rubber-sealed valves in favor of particle-free stainless steel
Everything in a compressed air system is interconnected. Contamination is rarely isolated, and particles can host moisture, which can harbor microbes, all exacerbated by oil residues.
Creating a Monitoring Plan
Understanding your system’s capabilities and unique compressed air contamination risks is the first step. Before setting stringent Class 1 requirements, conduct a risk assessment. Trace Analytics provides a Monitoring Plan Guide and a Risk Assessment Checklist to help you evaluate where, what, and how often to test.
Recommended testing points:
•At the point of use
•After the compressor (post-filtration)
•At any critical control points in between
Different sampling locations help identify where contamination originates.
Analysis Packages Tailored to Your Needs
Trace Analytics offers packages from Diagnostic (non-pass/fail) to Pro (specific ISO class compliance). Microbial testing packages, including Micro ID, can identify organisms down to genus and species. This can be invaluable, for example one case where yeast used in bread manufacturing was misidentified as contamination—Micro ID confirmed it was harmless.
Compressed air testing is an essential part of food safety, product quality, and audit readiness. As standards tighten and awareness grows, manufacturers must take proactive steps. With the right tools, testing protocols, and support, maintaining a safe and compliant compressed air system is entirely achievable.
To learn more or download the Risk Assessment and Monitoring Plan resources, visit Trace Analytics.