Original article featured on Compressed Air Best Practices.
Health care facilities utilize compressed air and gases in a variety of procedures for their patients. Regularly used in breathing, sedation, and medical devices, it is essential that compressed air is void of contaminants so that health care facilities can provide patient care with adequate safety. “Contamination of compressed air/gas, whether from ambient air, the air compressor itself, or the piping system, is a liability for health care facilities that can threaten patient health and safety, and ultimately cost the facility in malpractice lawsuits” (Stein 2019). Medical device manufacturers can help ensure safety and quality in compressed air systems through regular monitoring.
Compliance with NFPA99
Regulatory agencies ensure that proper safety protocols are established and that facilities are in compliance with these methods. “The Centers for Medicare and Medicaid Services (CMS), The Joint Commission (TJC), and local authorities having jurisdiction (AHJ), mandate that health care facilities ensure their compressed air and gas systems are compliant with NFPA 99” (Stein 2019).
Major Contaminants as Listed by NFPA 99
- Odor
- Water
- Carbon Monoxide (CO)
- Carbon Dioxide (CO2)
- Gaseous Hydrocarbons
- Halogenated Hydrocarbons
- Oil and particulates (viable and non-viable)
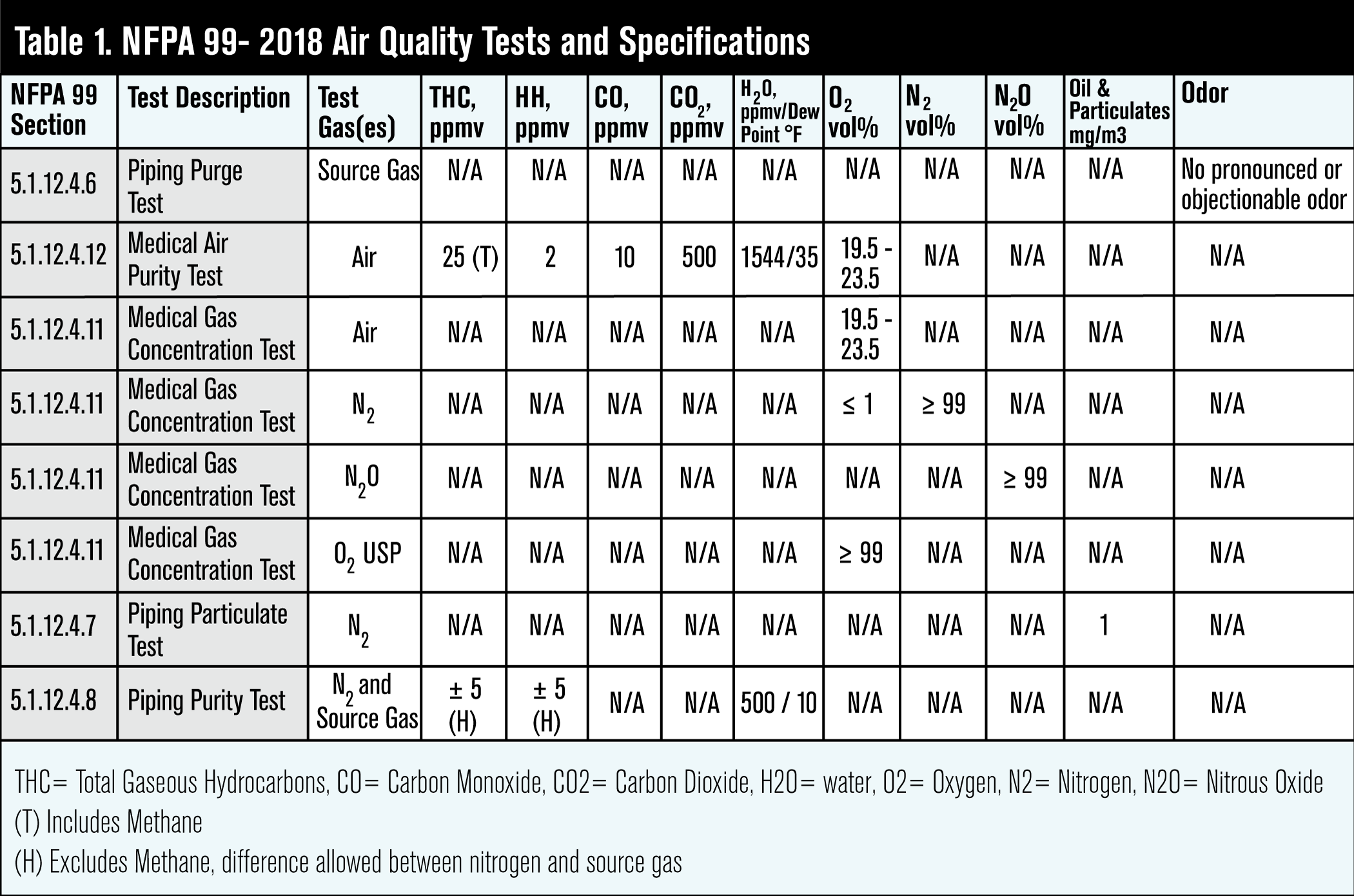
NFPA 99 lists the above as major contaminants that must be monitored regularly using its established purity limits outlined in Table 1.
Compressed Air And Gas Testing Analysis
Testing compressed air can be facilitated through the use of a third-party laboratory. As per NFPA 99 requirements, testing and analysis must be performed by a party other than the installer. When a system is not installed by in-house personnel, testing can be performed by an in-house American Society of Safety Engineer (ASSE) 6030-qualified employee. “ASSE 6030 qualified employees can complete verification testing per NFPA 99 using portable analyzers/monitors or in conjunction with a third-party laboratory” (Stein 2019).
Testing compressed air with portable analyzers provide facilities with the ease of producing quicker results in real-time. However, testing with an accredited laboratory “is often of higher accuracy and quality due to more complex techniques, instrumentation, and data analysis. Thus, a testing laboratory can sometimes provide clues as to the source of contamination through description/identification of particles, hydrocarbons, oils, etc” (Stein 2019).
Furthermore, third-party laboratory testing can be less expensive in the long-run. Portable analyzers run at a high cost due to the necessary maintenance and calibration that it requires. Ultimately, it is at the discretion of facilities to determine which testing method is appropriate for their use.
Utilizing Proper Air Treatments
NFPA 99 mandates the use of the proper air treatments for meeting their requirements. To combat water contamination, desiccant dryers are commonly used in health care facilities to remove water from the compressed air/gas as NFPA 99 requires dryers be designed to provide air at a maximum dewpoint of less than 32 °F at 50-55 psi. It is important to note that desiccant dryers can be the culprit for contamination as they release particulates into compressed air systems. To combat this issue, filters should be used in conjunction with desiccant dryers to help remove contamination present in ambient air or from the compressor itself. Point-of-use filters are crucial for ensuring that the air is not contaminated upon use, and regular testing ensures that these filters are functioning properly.
Ensure Patient Safety with Compressed Air Testing
To help ensure patient safety, health care facilities should be aware of the risks they face. Proper compliance with regulating agencies such as NFPA 99, regular compressed air and gas testing with a third-party laboratory, and ongoing monitoring of air treatments are paramount for medical grade applications.
Trace Analytics, LLC is an ISO 17025 accredited laboratory specializing in the analysis of compressed air and gas. The laboratory can provide testing for health care facilities who maintain their own equipment with an experienced medical gas and vacuum pipeline testing ASSE 6030 qualified employee. In addition, the laboratory tests to NFPA 99 standards and provides accurate results with a quick turnaround. They are a proud member of The Medical Gas Professional Healthcare Organization (MGPHO) and The National Fire Protection Association (NFPA).
References:
1. NFPA 99 Healthcare Facilities Code (2018)
“Verifying Compressed Air and Gas Safety. Safety and Quality in Medical Applications.” Compressed Air Best Practices, 2019. https://www.airbestpractices.com/standards/nfpa-99-medical-air/verifying-compressed-air-and-gas-safety-and-quality-medical-application