Original article published on International Food Safety and Quality Network.
Regulations protect the health and welfare of consumers. One such regulation is ISO 8573-7 which provides guidelines to manufacturers for compressed air testing for microorganisms. Failure to use a proper, validated sampling method to test compressed air can result in a false positive for microbial contamination.
In one case, a bakery in Illinois experienced false positive results requiring extensive re-testing, maintenance, and troubleshooting. Eventually, they discovered that they had allowed ambient air to mix with the compressed air sample. The tests were revealing microbes in the ambient air that did not exist in the compressed air (Parker Hannifin Corporation, 2014). Choosing the correct method and performing the sampling properly can ensure accurate results and, ultimately, save time and money.
Qualitative vs Quantitative Results
Qualitative microbial analyses only report the presence or absence of microorganisms, while quantitative analyses report the number of microbes in a specified volume. ISO 8573-7 calls for quantitative results and provides guidance on the appropriate equipment for sampling. Impaction samplers, which obtain results via total plate count per cubic meter, are specified in Annex B of the standard.
When choosing sampling equipment for microbial testing, it’s important to consider the goals of testing and the standards to which one is reporting. An inappropriate sampling method will not meet requirements and could result in a false positive result, or in the worst case scenario, not report dangerous microbial contamination that could force a recall or harm consumers.
Non-Validated Microbial Sampling Methods
The following methods are not validated as per ISO 8573-7 and will not provide quantitative results.
- Spraying compressed air/gas through a hose into a sterile bag that contains an open petri dish
- Spraying compressed air onto a sterile surface and stamping agar plate on that surface
- Spraying compressed air/gas directly onto the agar plate
- Spraying compressed air into sterile water and then analyzing water
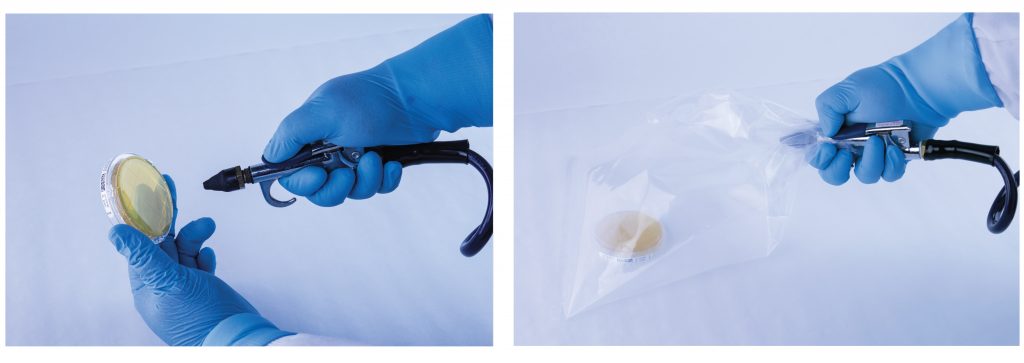
Testing in any of these manners will not provide you with results that can be relied upon (Sandoval, 2018).
- Methods cannot be validated
- Volume of tested air cannot be measured
- No controls are present
- Environmental microorganisms are likely to be introduced
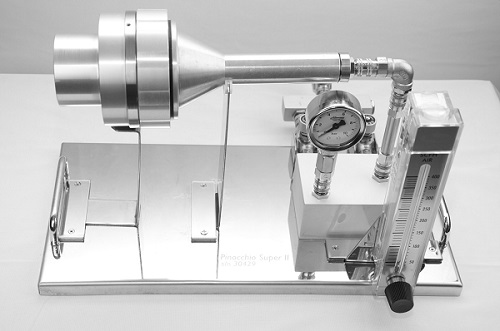
SAS Pinocchio Super II Impaction Sampler
At Trace Analytics LLC, we test compressed air for microbial contamination using the Pinocchio Super II Impaction Sampler. Specifically designed for compressed air testing, this piece of equipment is validated as per ISO 8573-7 and can provide quantitative results. Additionally, Trace Analytics has put together extensive instructions on proper testing procedures.
Aseptic technique, negative controls, validated sampling methods and a 3rd-party accredited laboratory used together in tandem help to ensure accurate results and a healthy compressed air system.
For more comprehensive information on microbial sampling techniques, read the original article by Maria Sandoval published on IFSQN.